|
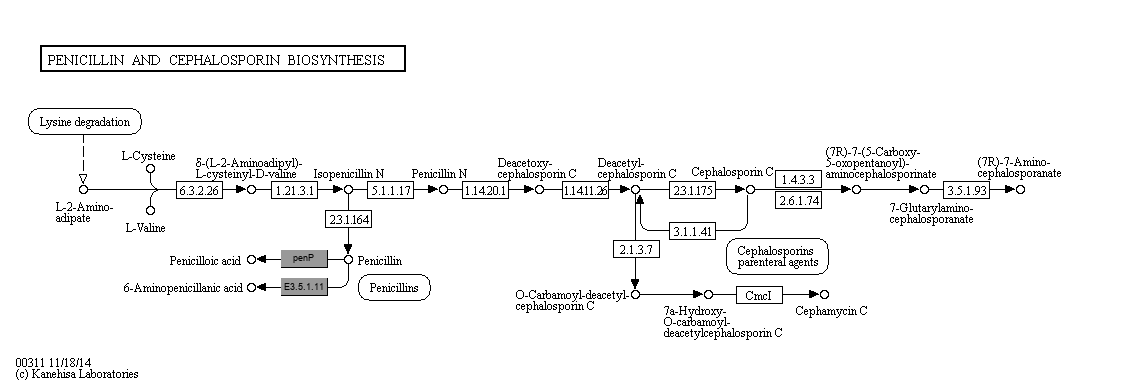
Penicillins (Penams) and cephalosporins (cephems) are beta-lactam antibiotics containing 6-aminopenicillanate (6-APA) and 7-aminocephalosporanate (7-ACA) nuclei, respectively. 6-APA and 7-ACA are key intermediates for a variety of semisynthetic penicillin and cephalosporin derivatives. Penicillins are produced only by fungi, while cephalosporins (including cephamycins) are produced by fungi and bacteria. Both antibiotics are synthesized from L-2-aminoadipate, L-cysteine and L-valine through a common pathway. It starts with the condensation of these three amino acids by the non-ribosomal peptide synthetase to form the tripeptide delta-(L-2-aminoadipyl)-L-cysteinyl-D-valine (ACV). The linear ACV tripeptide is then converted to bicyclic isopenicillin N by isopenicillin N synthase, in which the beta-lactam ring is formed. Isopenicillin N is the branch point of penicillin [MD:
M00672
] and cephalosporin [MD:
M00673
] pathways.
|
 Penicillin and cephalosporin biosynthesis - Reference pathway
Penicillin and cephalosporin biosynthesis - Reference pathway

 Penicillin and cephalosporin biosynthesis - Reference pathway
Penicillin and cephalosporin biosynthesis - Reference pathway