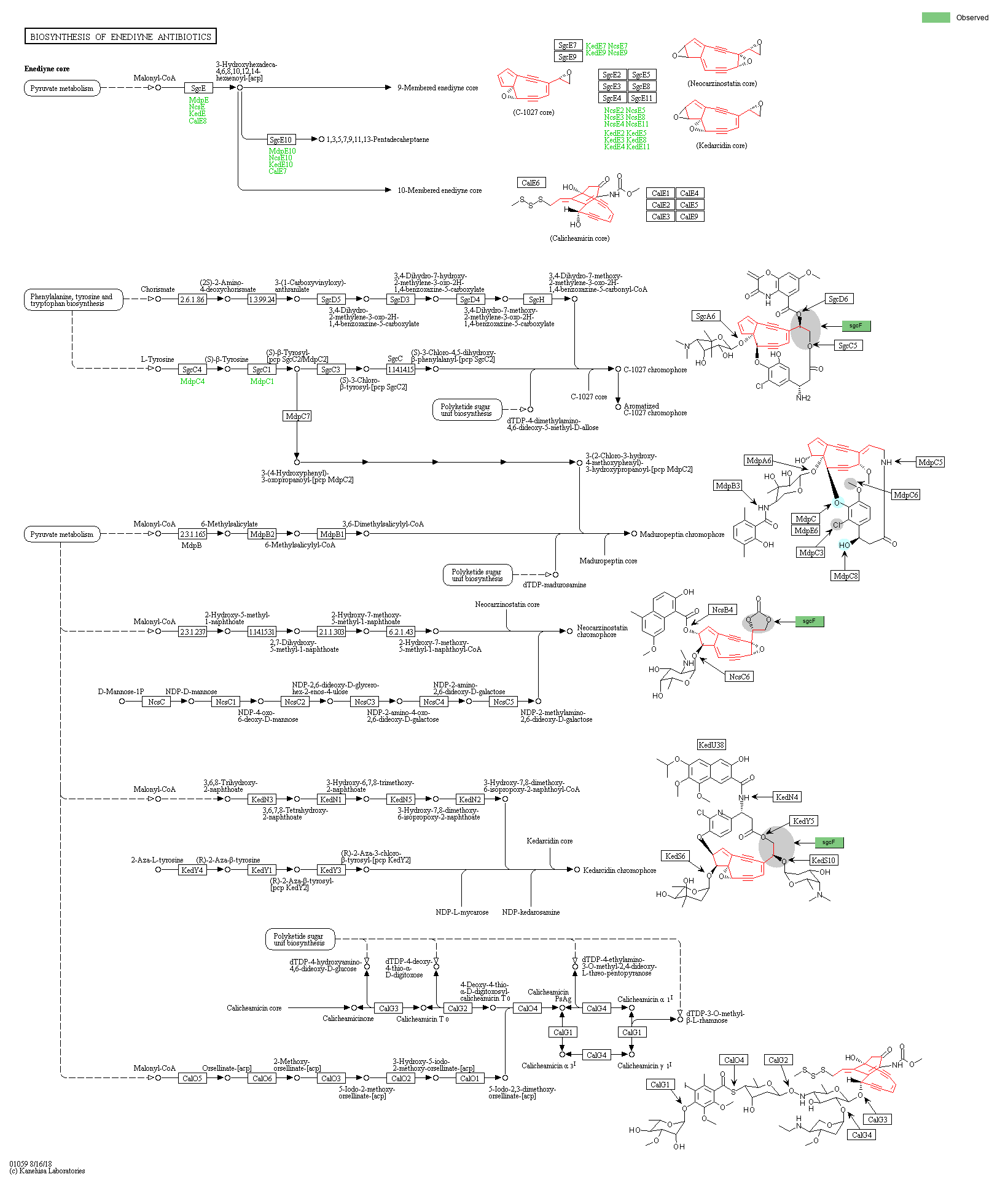
|
Enediyne natural products are potent antitumor antibiotics produced by a variety of Actinomycetes. Enediyne has a unique structure consisting of three building blocks: aromatic, sugar and enediyne core. The enediyne core contains two acetylenic groups conjugated to a double bond in a 9- or 10-membered ring, and it is synthesized by an iterative type I polyketide synthase and other tailoring proteins [MD:
M00824
M00825
]. The 10-membered enediyne such as calicheamicin also contains an allylic trisulfide group, which acts as a trigger for diradical formation. This diagram shows the biosynthesis of C-1027, maduropeptin, neocarzinostatin, kedarcidin and calicheamicin. Some aromatic moieties are synthesized via iterative type I polyketide synthases, and others are derived from chorismate and aromatic amino acids such as tyrosine and azatyrosine [MD:
M00829
M00830
M00831
M00834
M00826
M00827
M00828
M00832
]. Finally, the aromatic and sugar building blocks are attached to the enediyne core by acyltransferases, condensation enzymes and glucosyltransferases [MD:
M00833
].
|
 Biosynthesis of enediyne antibiotics - Reference pathway
Biosynthesis of enediyne antibiotics - Reference pathway

 Biosynthesis of enediyne antibiotics - Reference pathway
Biosynthesis of enediyne antibiotics - Reference pathway